19th century
John Dalton's union of atoms combined in ratios (1808)
In Amedeo Avogadro's famous 1811 paper "Essay on Determining the Relative Masses of the Elementary Molecules of Bodies", he essentially states, i.e. according to Partington's A Short History of Chemistry, that:[8]
| “ | The smallest particles of gases are not necessarily simple atoms, but are made up of a certain number of these atoms united by attraction to form a single molecule. | ” |
During his stay in Vercelli, Avogadro wrote a concise note (memoria) in which he declared the hypothesis of what we now call Avogadro's law: equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. This law implies that the relationship occurring between the weights of same volumes of different gases, at the same temperature and pressure, corresponds to the relationship between respective molecular weights. Hence, relative molecular masses could now be calculated from the masses of gas samples.
Avogadro developed this hypothesis in order to reconcile Joseph Louis Gay-Lussac's 1808 law on volumes and combining gases with Dalton's 1803 atomic theory. The greatest difficulty Avogadro had to resolve was the huge confusion at that time regarding atoms and molecules – one of the most important contributions of
In two papers outlining his "theory of atomicity of the elements" (1857–58), Friedrich August Kekulé was the first to offer a theory of how every atom in an organic molecule was bonded to every other atom. He proposed that carbon atoms were tetravalent, and could bond to themselves to form the carbon skeletons of organic molecules.
In 1861, an unknown Vienna high-school teacher named Joseph Loschmidt published, at his own expense, a booklet entitled Chemische Studien I, containing pioneering molecular images which showed both "ringed" structures as well as double-bonded structures, such as:[12]
Loschmidt also suggested a possible formula for benzene, but left the issue open. The first proposal of the modern structure for benzene was due to Kekulé, in 1865. The cyclic nature of benzene was finally confirmed by the crystallographer Kathleen Lonsdale. Benzene presents a special problem in that, to account for all the bonds, there must be alternating double carbon bonds:
Benzene molecule with alternating double bonds
| “ | An atom is a body which cannot be cut in two; a molecule is the smallest possible portion of a particular substance. | ” |
In 1874, Jacobus Henricus van 't Hoff and Joseph Achille Le Bel independently proposed that the phenomenon of optical activity could be explained by assuming that the chemical bonds between carbon atoms and their neighbors were directed towards the corners of a regular tetrahedron. This led to a better understanding of the three-dimensional nature of molecules.
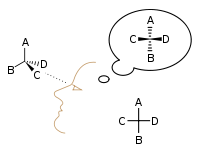
Emil Fischer developed the Fischer projection technique for viewing 3-D molecules on a 2-D sheet of paper:
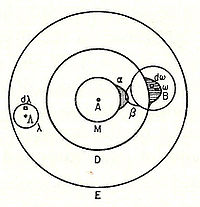
In 1898, Ludwig Boltzmann, in his Lectures on Gas Theory, used the theory of valence to explain the phenomenon of gas phase molecular dissociation, and in doing so drew one of the first rudimentary yet detailed atomic orbital overlap drawings. Noting first the known fact that molecular iodine vapor dissociates into atoms at higher temperatures, Boltzmann states that we must explain the existence of molecules composed of two atoms, the “double atom” as Boltzmann calls it, by an attractive force acting between the two atoms. Boltzmann states that this chemical attraction, owing to certain facts of chemical valence, must be associated with a relatively small region on the surface of the atom called the sensitive region.
Boltzmann states that this "sensitive region" will lie on the surface of the atom, or may partially lie inside the atom, and will firmly be connected to it. Specifically, he states “only when two atoms are situated so that their sensitive regions are in contact, or partly overlap, will there be a chemical attraction between them. We then say that they are chemically bound to each other.” This picture is detailed below, showing the α-sensitive region of atom-A overlapping with the β-sensitive region of atom-B:[15]




No comments:
Post a Comment